Leptomeningeal Metastases Market to Exhibit Growth at a Paltry CAGR of 1.9% During the Forecast Period (2025–2034) | DelveInsight
The leptomeningeal metastases market is projected to grow steadily due to rising cancer incidence and the increasing prevalence of central nervous system complications in advanced malignancies. Additionally, the expected launch of therapies such as Rhenium-186 obisbemeda (REYOBIQ), Paxalisib, HSV G2072, AZD1390, and others will further propel the leptomeningeal metastases market growth.
New York, USA, Sept. 09, 2025 (GLOBE NEWSWIRE) -- Leptomeningeal Metastases Market to Exhibit Growth at a Paltry CAGR of 1.9% During the Forecast Period (2025–2034) | DelveInsight
The leptomeningeal metastases market is projected to grow steadily due to rising cancer incidence and the increasing prevalence of central nervous system complications in advanced malignancies. Additionally, the expected launch of therapies such as Rhenium-186 obisbemeda (REYOBIQ), Paxalisib, HSV G2072, AZD1390, and others will further propel the leptomeningeal metastases market growth.
DelveInsight’s Leptomeningeal Metastases Market Insights report includes a comprehensive understanding of current treatment practices, emerging leptomeningeal metastases drugs, market share of individual therapies, and current and forecasted leptomeningeal metastases market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
Leptomeningeal Metastases Market Summary
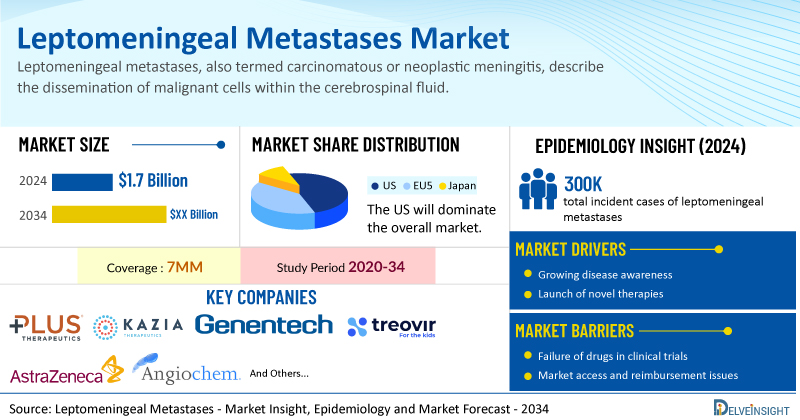
- The total leptomeningeal metastases treatment market size was USD 1.7 billion in 2024, and it is expected to grow positively by 2034 in the leading markets (the US, EU4, UK, and Japan).
- The United States accounts for the largest market size of leptomeningeal metastases, i.e, 60%, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- In 2024, the total incident cases of leptomeningeal metastases were ~300K in the leading markets (the US, EU4, UK, and Japan).
- Key leptomeningeal metastases companies, including Plus Therapeutics, Kazia Therapeutics, Genentech, Treovir, AstraZeneca, Angiochem, and others, are actively working on innovative leptomeningeal metastases drugs.
- Some of the key leptomeningeal metastases therapies in clinical trials include Rhenium-186 obisbemeda (REYOBIQ), Paxalisib, HSV G2072, AZD1390, ANG1005, and others. These novel leptomeningeal metastases therapies are anticipated to enter the leptomeningeal metastases market in the forecast period and are expected to change the market.
Discover which leptomeningeal metastases medications are expected to grab the market share @ Leptomeningeal Metastases Market Report
Key Factors Driving the Growth of the Leptomeningeal Metastases Market
Rising Incidence of Central Nervous System Metastases
Advancements in systemic cancer therapies have improved overall survival rates, leading to a higher prevalence of CNS metastases, including leptomeningeal metastases. As patients live longer, the occurrence of leptomeningeal metastases as a complication has increased, thereby expanding the patient population requiring specialized treatment. In 2024, the total incident cases of leptomeningeal metastases were approximately 120K in the US, reflecting its significant disease burden and the pressing need for improved diagnostic tools and more effective treatments.
Significant Unmet Clinical Needs
Currently, there are no FDA-approved therapies specifically for leptomeningeal metastases, and the condition is often diagnosed late due to the lack of effective screening methods. This gap presents a substantial opportunity for the development of targeted treatments that can address the unique challenges of leptomeningeal metastases, such as the blood–brain barrier and limited drug penetration into the cerebrospinal fluid.
Emerging Leptomeningeal Metastases Therapies
The development of novel leptomeningeal metastases therapies is gaining momentum. For instance, Plus Therapeutics is advancing Rhenium (186Re) Obisbemeda, a radiotherapeutic targeting leptomeningeal metastases, currently undergoing clinical trials. Additionally, antibody-drug conjugates like Patritumab deruxtecan (Daiichi Sankyo) have shown promising activity in treating leptomeningeal metastases, offering hope for patients with limited options.
Leptomeningeal Metastases Market Analysis
Despite the substantial disease burden, there are currently no therapies approved explicitly for leptomeningeal metastases. Treatment remains largely palliative and multimodal, aimed at symptom relief using off-label agents such as methotrexate, cytarabine, temozolomide, capecitabine, topotecan, and lapatinib, often in combination with radiation therapy. These strategies focus on slowing disease progression and preserving neurological function.
In 2024, the US accounted for nearly 60% of the leptomeningeal metastases market, driven by widespread use of systemic, targeted, intrathecal, and radiation-based interventions. This dominance is supported by favorable reimbursement policies, rapid uptake of novel treatments, and a healthcare system that facilitates high-cost, aggressive therapy approaches.
Pharmaceutical involvement in leptomeningeal metastases has mostly been supportive, with companies contributing investigational drugs or partial funding to academic-led trials rather than leading full-scale development programs. Notable exceptions include Plus Therapeutics, which is actively developing REYOBIQ, a targeted radiotherapeutic for leptomeningeal metastases, and Angiochem, which had advanced ANG1005, a peptide-drug conjugate capable of crossing the blood–brain and blood–CSF barriers. However, its development is currently on hold.
The emerging leptomeningeal metastases treatment landscape has encountered significant challenges. For example, Y-mAbs Therapeutics’ Omburtamab, a radiolabeled monoclonal antibody targeting B7-H3 in CNS tumors and leptomeningeal metastases, completed pivotal trials but received a Complete Response Letter (CRL) from the FDA in 2022, citing insufficient clinical benefit and concerns regarding study design.
Learn more about the leptomeningeal metastases treatment options @ Leptomeningeal Metastases Treatment Guidelines
Leptomeningeal Metastases Competitive Landscape
Some of the key leptomeningeal metastases drug clinical trials include REYOBIQ (Plus Therapeutics), Paxalisib (Kazia Therapeutics/Genentech), HSV G2072 (Treovir), AZD1390 (AstraZeneca), ANG1005 (Angiochem), and others.
REYOBIQ (rhenium Re186 obisbemeda) is a novel injectable radiotherapy engineered to deliver high-dose, targeted radiation directly to tumors in the central nervous system, emphasizing safety, effectiveness, and ease of use. By harnessing the distinct properties of rhenium-186, its short half-life, beta emissions for tumor destruction, and gamma emissions for real-time imaging, REYOBIQ presents a promising approach for CNS-focused treatments. It is currently being investigated for leptomeningeal metastases in the ongoing ReSPECT-LM clinical trials.
Paxalisib (GDC-0084) is an investigational therapy being developed to treat various brain cancers. It functions as a brain-penetrant inhibitor of the PI3K/Akt/mTOR signaling pathway, which is essential for regulating cell growth and division. Paxalisib’s key advantage lies in its ability to cross the blood-brain barrier, allowing effective drug delivery to brain tissue, a critical factor in targeting central nervous system malignancies.
The anticipated launch of these emerging leptomeningeal metastases therapies are poised to transform the leptomeningeal metastases market landscape in the coming years. As these cutting-edge leptomeningeal metastases therapies continue to mature and gain regulatory approval, they are expected to reshape the leptomeningeal metastases market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for leptomeningeal metastases, visit @ Leptomeningeal Metastases Management
Recent Developments in the Leptomeningeal Metastases Market
- In July 2025, Plus Therapeutics stated that it will unveil findings from its ReSPECT-LM clinical trial and lead a sponsored educational symposium during the upcoming SNO/ASCO CNS Metastases Conference, taking place from August 14 to 16, 2025.
- In April 2025, the Phase I ReSPECT-LM trial of REYOBIQ (Rhenium Obisbemeda) in leptomeningeal metastases showed a dose-dependent increase in drug delivery, reaching 253 Gy in Cohort 5. Neuroimaging data from 16 patients showed a 31% partial response rate and a 75% clinical benefit rate. Physician assessments in 14 patients reported a 14% response rate and 86% clinical benefit.
- In October 2024, at the 66th Annual Meeting of ASTRO, Kazia Therapeutics shared Phase I data revealing that a 45 mg dose of paxalisib, when combined with radiotherapy, resulted in a 67% partial response rate in patients with brain or leptomeningeal metastases harboring PI3K mutations. Notably, more than two-thirds of patients treated at the maximum tolerated dose (MTD) showed intracranial responses—exceeding historical benchmarks for radiation therapy alone.
What is Leptomeningeal Metastases?
Leptomeningeal metastases, also termed carcinomatous or neoplastic meningitis, describe the dissemination of malignant cells within the cerebrospinal fluid. These cells may arise from primary central nervous system tumors, such as drop metastases, or from distant cancers that spread through the bloodstream.
Leptomeningeal Metastases Epidemiology Segmentation
The leptomeningeal metastases market report is a comprehensive and specialized analysis, offering in-depth epidemiological insights for the study period 2020–2034 across the leading markets. In 2024, the incidence of cases of leptomeningeal metastases was approximately 300K in the 7MM, reflecting a significant clinical burden. As per DelveInsight analysis, the US showed the highest number of incident cases of leptomeningeal metastases, accounting for approximately 40% of the incident cases of leptomeningeal metastases in the 7MM in 2024.
The leptomeningeal metastases market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets, segmented into:
- Total Incident Cases of Leptomeningeal Metastases
- Incident Cases of Leptomeningeal Metastases by Cancer Type
- Incident Cases of Leptomeningeal Metastases by Risk Type
- Gender-specific Cases of Leptomeningeal Metastases
- Leptomeningeal Metastases Treated Patient Pool
Download the report to understand which factors are driving leptomeningeal metastases epidemiology trends @ Leptomeningeal Metastases Treatment Algorithm
| Leptomeningeal Metastases Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Leptomeningeal Metastases Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Leptomeningeal Metastases Market CAGR | 1.9% |
| Leptomeningeal Metastases Market Size in 2024 | USD 1.7 Billion |
| Key Leptomeningeal Metastases Companies | Plus Therapeutics, Kazia Therapeutics, Genentech, Treovir, AstraZeneca, Angiochem, and others |
| Key Leptomeningeal Metastases Therapies | Rhenium-186 obisbemeda (REYOBIQ), Paxalisib, HSV G2072, AZD1390, ANG1005, and others |
Scope of the Leptomeningeal Metastases Market Report
- Leptomeningeal Metastases Therapeutic Assessment: Leptomeningeal Metastases current marketed and emerging therapies
- Leptomeningeal Metastases Market Dynamics: Conjoint Analysis of Emerging Leptomeningeal Metastases Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Leptomeningeal Metastases Market Unmet Needs, KOL’s views, Analyst’s views, Leptomeningeal Metastases Market Access and Reimbursement
Discover more about leptomeningeal metastases drugs in development @ Leptomeningeal Metastases Clinical Trials
Table of Contents
| 1 | Leptomeningeal Metastases Market Key Insights |
| 2 | Leptomeningeal Metastases Market Report Introduction |
| 3 | Leptomeningeal Metastases Market Overview at a Glance |
| 3.1 | Market Share (%) Distribution of Leptomeningeal Metastases by Therapies in 2024 in the 7MM |
| 4 | Epidemiology and Market Methodology |
| 5 | Executive Summary |
| 6 | Key Events |
| 7 | Leptomeningeal Metastases Disease Background and Overview |
| 7.1 | Introduction |
| 7.2 | Leptomeningeal Metastases Signs and Symptoms |
| 7.3 | Leptomeningeal Metastases Causes |
| 7.4 | Leptomeningeal Metastases Risk Factors |
| 7.5 | Prognosis |
| 7.6 | Pathophysiology |
| 7.7 | Complications |
| 7.8 | Leptomeningeal Metastases Diagnosis |
| 8 | Leptomeningeal Metastases Treatment and Management |
| 9 | Leptomeningeal Metastases Epidemiology and Patient Population |
| 9.1 | Key Findings |
| 9.2 | Assumptions and Rationale |
| 9.3 | Total Incident Cases of Leptomeningeal Metastases in the 7MM |
| 9.4 | The United States |
| 9.4.1 | Total Incident Cases of Leptomeningeal Metastases in the United States |
| 9.4.2 | Incident cases of Leptomeningeal Metastases, by Cancer Type in the United States |
| 9.4.3 | Leptomeningeal Metastases, by Risk Type in the United States |
| 9.4.4 | Gender-specific Cases of Leptomeningeal Metastases in the United States |
| 9.4.5 | Leptomeningeal Metastases Treated Patient Pool in the United States |
| 9.5 | EU4 and the UK |
| 9.6 | Japan |
| 10 | Leptomeningeal Metastases Patient Journey |
| 11 | Emerging Leptomeningeal Metastases Therapies |
| 11.1 | Key Cross Competition |
| 11.2 | REYOBIQ (rhenium Re186 obisbemeda): Plus Therapeutics |
| 11.2.1 | Product Description |
| 11.2.2 | Other Developmental Activities |
| 11.2.3 | Ongoing Clinical Development activity |
| 11.2.4 | Safety and Efficacy |
| 11.2.5 | Analyst Views |
| 11.3 | Paxalisib: Kazia Therapeutics/Genentech |
| 12 | Leptomeningeal Metastases Market - Seven Major Market Analysis |
| 12.1 | Key Findings |
| 12.2 | Leptomeningeal Metastases Market Outlook |
| 12.3 | Key Leptomeningeal Metastases Market Forecast Assumptions |
| 12.4 | Total Market Size of Leptomeningeal Metastases in the 7MM |
| 12.5 | Market Size of Leptomeningeal Metastases by Therapies in the 7MM |
| 12.6 | Leptomeningeal Metastases Market Size in the United States |
| 12.6.1 | Total Market Size of Leptomeningeal Metastases in the United States |
| 12.6.2 | Market Size of Leptomeningeal Metastases by Therapies in the United States |
| 12.7 | Leptomeningeal Metastases Market Size in EU4 and the UK |
| 12.8 | Leptomeningeal Metastases Market Size in Japan |
| 13 | KOL Views on Leptomeningeal Metastases |
| 14 | Leptomeningeal Metastases Market SWOT Analysis |
| 15 | Leptomeningeal Metastases Market Unmet Needs |
| 16 | Leptomeningeal Metastases Market Access and Reimbursement |
| 16.1 | United States |
| 16.2 | EU4 and the UK |
| 16.3 | Japan |
| 17 | Bibliography |
| 18 | Leptomeningeal Metastases Market Report Methodology |
Related Reports
Leptomeningeal Metastases Clinical Trial Analysis
Leptomeningeal Metastases Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key leptomeningeal metastases companies, including AstraZeneca, Kazia Therapeutics, Plus Therapeutics, among others.
Glioblastoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key glioblastoma companies, including Bayer, Chimerix, Aivita Biomedical, Denovo Biopharma, Northwest Therapeutics, VBL Therapeutics, Laminar Pharmaceuticals, MedImmune, DNAtrix, Immunomic Therapeutics, Imvax, MimiVax, CNS Pharmaceuticals, Epitopoietic Research Corporation (ERC), Istari Oncology, SonALAsense, Kintara Therapeutics, Bristol Myers Squibb, Medicenna Therapeutics, BioMimetix, Eisai, Merck Sharp & Dohme, Kazia Therapeutics, Oblato, Genenta Science, Enterome, Inovio Pharmaceuticals, Karyopharm Therapeutics, Forma Therapeutics, VBI Vaccines, TME Pharma, among others.
Glioblastoma Clinical Trial Analysis
Glioblastoma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key glioblastoma companies, including Denovo Biopharma, AstraZeneca, Pfizer, Chimerix, Bristol-Myers Squibb, Orbus Therapeutics, Northwest Biotherapeutics, Day One Biopharmaceuticals, AiVita Biomedical, Ascletis Pharma Inc., Bristol Myers Squibb, Kazia Therapeutics, HebaBiz Biotech, Biohaven Pharmaceuticals, Vigeo Therapeutics, Hoffman-La-Roche, TVAX Biomedical, Laminar Pharmaceuticals, Kintara Therapeutics, Medicenna Therapeutics, Symphogen A/S, MimiVax, Incyte Corporation, Istari Oncology, Immunomic Therapeutics, Celgene, Sanofi, Merck Sharp & Dohme LLC, Oblato, Inc., GlaxoSmithKline, NuvOx Pharma, Epitopoietic Research Corporation, AnHeart Therapeutics, DNAtrix, Arog Pharmaceuticals, CANbridge Pharmaceuticals, Jiangsu Hengrui Medicine, BPGbio, Inc., BioMimetix, CNS Pharmaceuticals, among others.
Brain Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key brain cancer companies, including Y-mAbs Therapeutics Inc., Shimadzu Corporation, Bristol-Myers Squibb, Bayer AG, AstraZeneca plc, Johnson & Johnson Inc., Merck & Co., Pfizer Inc., F. Hoffmann-La Roche Ltd, Dr. Reddy's Laboratories Ltd, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
